 The study used a a simple loofah, powered by silver nano-donuts
The study used a a simple loofah, powered by silver nano-donutsThe simple bathroom loofah could be the future for green hydrogen production, a new study has found.
Researchers at Bournemouth University combined a sample of nature’s bath sponge with some nano-sized silver donuts to create a catalyst that splits water into hydrogen and oxygen when an electrical current passes through it.
The findings, published in the journal Applied Materials and Interfaces, could offer a low-cost and sustainable means to produce hydrogen from renewable materials.
Increasing hydrogen production is a vital part of the UK Government’s energy strategy and ambition to achieve net zero.
“Hydrogen as a fuel stands out as a key solution in meeting the rising energy demand, explained Dr Amor Abdelkader, Associate Professor in Advanced Materials at Bournemouth University. “But the most common method of producing it, by ‘cracking’ hydrocarbon gasses, is heavily reliant on fossil fuels. It is essential that we reduce the carbon footprint in hydrogen production for a safer and sustainable future,” he added.
In recent years, using electrocatalysts to split water has given industry another way to produce hydrogen more sustainably, however current methods use platinum as a catalyst which is extremely expensive.
The Bournemouth University team, led by Dr Abdelkader set out to find a cheaper, lighter and more efficient catalyst to split water.
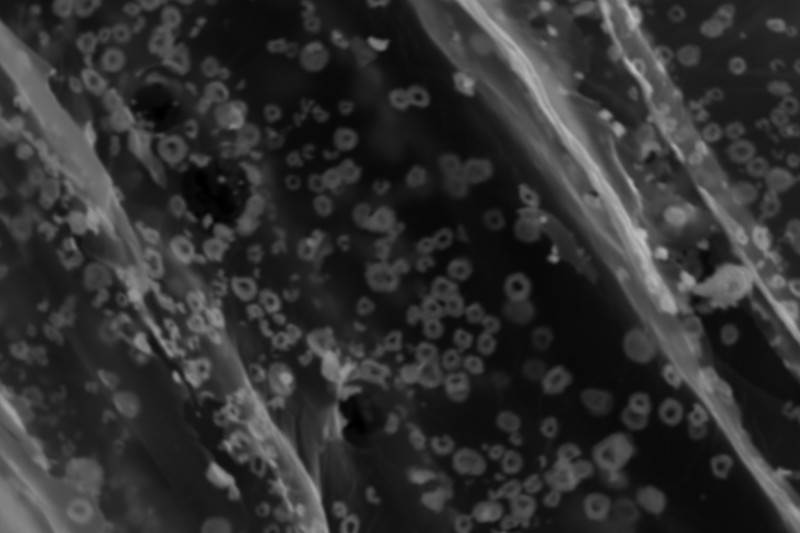
“We chose a loofah for the body because it contains carbon and nitrogen which are ideal ingredients for a catalyst and its natural tube-like structure is perfect for adding other ingredients," said Chirag Ratwani, a Bournemouth University researcher and leading member of the team.
“Silver also has important catalytic properties, so we developed a number of tiny rings – like nano donuts – from silver and inserted them into the loofah’s micro tubes,” Chirag added.
To further enhance the performance of the silver donuts, the team gave them a special sugar coating by removing some of their atoms. This created imbalances in their atomic structure which speeds up the splitting process.
After soaking the donut-powered loofah catalyst in water, the team connected it to an electrode and passed a current through it which successfully split the water and released hydrogen.
“This method could significantly impact the development of sustainable and cost-effective water-splitting systems and contribute not only to a cleaner environment but also to better energy sustainability,” said Shadeepa Karunarathne, a PhD Student at Bournemouth University.
“Less than 50% of our catalyst was metal, the rest was nature’s bath sponge which made it lighter and lower cost as well as more efficient," Dr Abdelkader said. "Loofahs are becoming increasingly popular again in our homes because people prefer a natural, renewable product over plastic. Now we can see that they could have further environmental benefits by lowering the carbon footprint of the energy industry,” he concluded.



